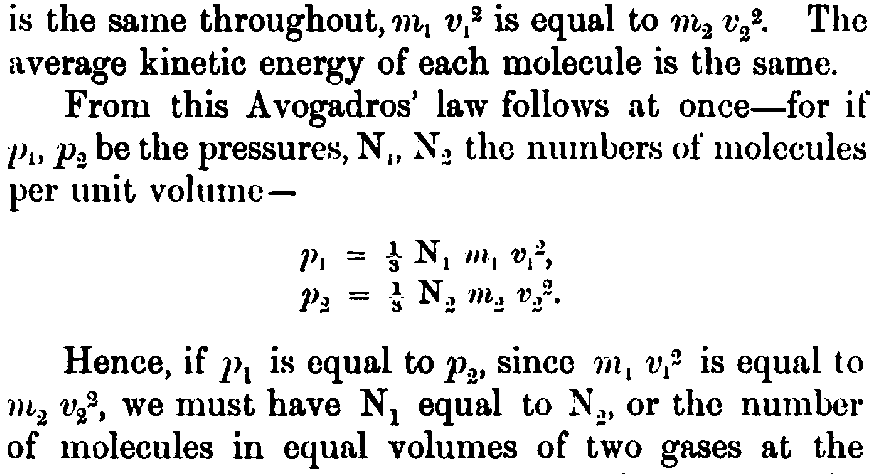Library of Formatting Examples:Italics/54A
Jump to navigation
Jump to search
| Distributed Proofreaders: Activity Hub · Wiki · Forums · Blog | ||
| << (Italics/53A) | Library of Formatting Examples Italics |
(Italics/55A) >> |
Correctly formatted text
is the same throughout, <i>m</i>_{1} <i>v</i>_{1}^2 is equal to <i>m</i>_{2} <i>v</i>_{2}^2. The average kinetic energy of each molecule is the same. From this Avogadros' law follows at once--for if <i>p</i>_{1}, <i>p</i>_{2} be the pressures, N_{1}, N_{2} the numbers of molecules per unit volume-- /* <i>p</i>_{1} = 1/3 N_{1} <i>m</i>_{1} <i>v</i>_{1}^2, <i>p</i>_{2} = 1/3 N_{2} <i>m</i>_{2} <i>v</i>_{2}^2. */ Hence, if <i>p</i>_{1} is equal to <i>p</i>_{2}, since <i>m</i>_{1} <i>v</i>_{1}^2 is equal to <i>m</i>_{2} <i>v</i>_{2}^2, we must have N_{1} equal to N_{2}, or the number of molecules in equal volumes of two gases at the
Subscripts and superscripts
The subscripts and superscripts are upright, not in italics, so they go outside the tags.